Soil Organic Matter
© 2007 Donald G. McGahan (aka soilman) All Rights Reserved
The original source of all organic matter is from plant tissue. The energy to fix the carbon into organic matter originates, mostly, from the sun. Microbes decompose (respiration) organic matter to obtain energy to sustain their life processes. The energy content of organic matter held in one hectare of soil with 4% organic matter is equivalent to 225 barrels of oil.
Globally soils hold considerable organic matter. Soil also has a considerable amount of carbonates and while carbonates are not organic matter they are largely present do to biological processes.
Photosynthesis ⥂ Metabolism (e.g. respiration)
6 CO₂ + H₂O + Energy ⇄ C₆H₁₂O₆ + O₂↑
Note: the arrow left and right is retained here to emphasize that carbon that is fixed into plant structures is subject to decomposition. The emphasis above is that the primary productivity exceeds metabolism of the product.
The inorganic carbon (IC) contained in soil carbonates is equally as considerable as the soil organic matter carbon (OC). Together the carbon content from these two soil pools, ICpool + OCpool, can constitute over twice the amount of carbon in the atmosphere and hydrosphere combined making this soil carbon pool of critical importance for global carbon processes and outcomes such as greenhouse effects impacting global warming.
Plant tissue is overwhelmingly the source of Soil Organic Matter (SOM), or humus, but the tissue of living animals in the soil contributes.
- Humus
- The stable, complex, and rather resistant mixture of dark brown to black, colloidal, organic substances that accumulates as a by-product of microbial decomposition and synthesis of plant or animal residues added to soil. The term is often used synonymously with soil organic matter.
Six groups of organic matter material:
- Plants
- Plant roots
- Dead and decaying residues of plants (termed litter when on the soil surface)
- Decay agents, mainly microorganism but including a wide array of soil microorganisms and soil fauna
- Colloidal decayed residues
- Water-soluble organic compounds
Plant Tissue ⟶ Microbes ⟶ Humus
Animal Tissue ⟶ Microbes ⟶ Humus
The microbial communities are harvesting the energy, metabolism, stored in the compounds. To simplify the reaction is shown with sugar below.
C₆H₁₂O₆ + O₂ ⇄ 6 CO₂↑ + H₂O + Energy
Organic Matter + Oxygen ⇄ Carbon Dioxide↑ + Water + Energy
Opposite of the building of organic matter by photosynthesis.
Metabolism (e.g. respiration) ⇄ Photosynthesis
Metabolism rarely encounters only something as simple as the sugar (C₆H₁₂O₆) depicted above. Thus the residence time of the carbon in the soil can be long lived. Organisms will continue decomposition to attain C and the other remaining nutrients needed for their life cycle. Composition of the carbon containing compounds impacts soil nutrient levels for plants and for the soil environment at large.
Plant mass is 60% to 90% water. The remaining dry matter is dominated by carbon, oxygen, and hydrogen.
| Element | Amount |
|---|---|
| carbon | 42% |
| oxygen | 42% |
| hydrogen | 7 to 8% |
| nitrogen | 1% |
| ash† | 8% |
†Plant residue ash is composed of inorganic elements such as Ca, Mg, K (Ash as in the stuff that accumulates at the bottom of the fireplace).
Most soils have less than 5% organic matter by weight. The amount of organic matter found in a soil is a balance between organic matter productionproduction and decomposition.
What factors influence organic matter production and decomposition rates?
| Compounds | decomposition rate |
|---|---|
| Sugar, starch, and simple proteins | Rapid |
| Crude proteins | ↑ |
| Hemicellulose | |
| Cellulose | |
| Fats, waxes, and tannins | ↓ |
| Lignin | Very Slow |
When we describe a soils morphology we assign the soils organizational packets (horizons) and if they are dominantly organic material we assign a master horizon designator of ‘O’ We then can add more meaning to the ‘O’ by adding subordinate designators.
- O horizon
- a surface layer dominated by organic materials (> 20% organic carbon; by weight.)
- Oi
- slightly decomposed organic matter. The `i' is an abbriviation for fibric and morphologically this means that the describer can still identify the original plant and animal remains
- Oe
- the organic matter is intermediately decomposed. The `e' is an abbreviation for hemic and morphologically this means that the describer can see some identifiable plant parts but their decomposition is in-between fibric (i) and sapric (a).
- Oa
- the organic matter is highly decomposed. The `a' is an abbreviation for sapric and morphologically this means that the describer can not identify the original source of the organic material.
Fractionation of the Soil Organic Matter
The larger sized SOM fraction (call these the macroorganics) in soils can be separated by sieving or placing the sample in water. The organic matter is much lower in density and much of it tends to float while the mineral fraction will sink.
The microorganics SOM is further divided. One way to look at the microorganics is separating dissolved verses not-dissolved. Subtracting the macroorganics and the dissolved microorganics is a working definition of humus.
- Humus
- The well decomposed, more or less stable part of the organic matter in mineral soils that accumulates as a by-product of decomposition of plant or animal residues (added to soil). The term is often used synonymously with soil organic matter.
- Humus is a complex and rather resistant (stable) mixture of dark brown to black colloidal organic substances that results from microbial decomposition and synthesis.
The first fractionation of humus is separating the identifiable compounds from the unidentifiable. Next the humus is treated with strong NaOH and the humus that is insoluble in that alkaline solution is termed humin. The solubilized fraction is then acidified and the insoluble portion is termed humic acid. The remaining soluble fraction of organic matter is called fulvic acid.
Because it is complex it is operationally defined as outlined in the following diagram.
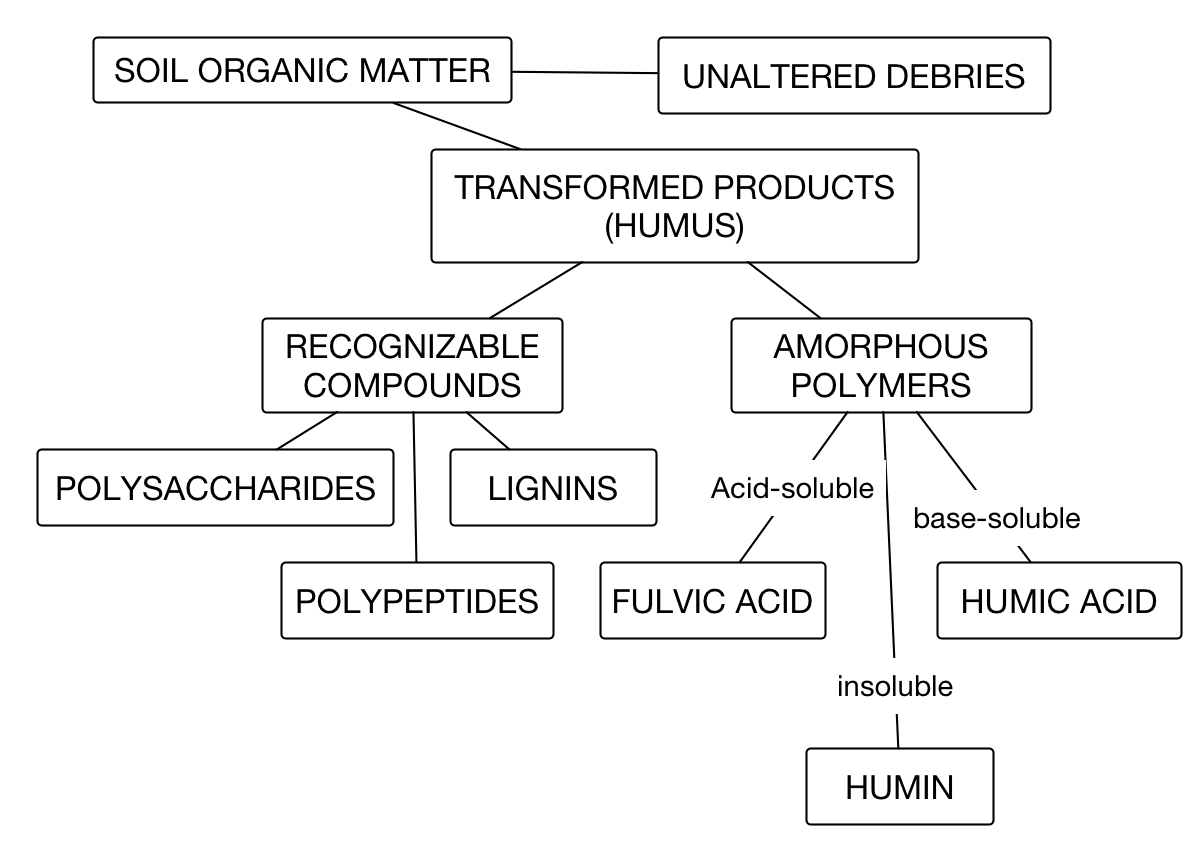
The Identifiable and relatively unaltered organic debris are not considered to be as chemically and physically reactive as the humus. Humus has lost all the visible features of the organics from which it decomposed from. Its further decomposition is much slower and it is said to be more stable. It is dark-colored and may be a by-product of decomposition of plant or animal residues added to soil. The term, humus, is often used synonymously with soil organic matter.
It has been useful to further separate the Humus into humic acid, fulvic acid, and humin.
- Humic acid
- consists of organic materials that are soluble in alkaline solution but insoluble on acidification of the alkaline extracts. This component is an acid because hydrogen ions at the surface of these complex molecules can be displaced by other cations such as calcium, magnesium, potassium, or sodium. By definition, any compound that participates in contributions of hydrogen to solution is an acid.
- Fulvic acid
- compounds are soluble in an alkaline solution and remained in solution when the alkaline extract is acidified. Sodium hydroxide (NaOH) in solution is an example of alkaline solution that extracts humic and fulvic acids.
- Humin
- is operationally defined fraction of humus that is insoluble in the alkaline solution.
The table of Humic Substances Properties demonstrates a trend toward higher weight compounds from fulvic acid through humic acid to humin. This continuum toward weight compounds likely indicates increasing resemblance to the complex aromatic polymer lignin.
| Fulvic Acid | Humic Acid | Humina | |
|---|---|---|---|
| Molecular Wt. | 1000–5000 | 10,000–100,000 | > 100,000 |
| % C | 42–47 | 51–62 | > 62 |
| % O | 45–50 | 31–36 | < 30 |
| % N | 2.0–4.1 | 3.6–5.5 | > 5 |
| Acid content (moles/kg)b | 14 | 5 | < 5 |
| aValues for humin are uncertain because of difficulty in separating this fraction from the mineral particles to perform the elemental analysis. | |||
| bAcid content is equivalent to the potential cation exchange capacity once the acidity is neutralized by alkali. | |||
More About Humus
- Humus decomposes slowly and colors soils brown or black.
- Humus has two properties in common with clay; it is highly charged, and it has a large surface area per unit mass (Table below: Surface area varies with the type of clay). Thus it is very reactive in soils.
- Humus is also an aggregator.
- Humus contains many branching chains of R-COOH and R-OH that can gain or lose hydrogen ions depending upon pH (pH dependent charge).
The most biologically active fraction of the SOM is the microbial fraction.
The most chemically reactive fraction of the SOM is the microorganic fraction.
- Composition of organic matter (grasslands vs forest)
- See Table of retaliative decomposability of organic compounds and consider that woody vegetation produces more compounds that are slow and very slow too decompose.
- Environmental Factors
- Temperature
- Activity generally increases with temperature until molecules become denatured at high temperature.
- Activity generally decreases slowly below 25 ℃ and dramatically below 15℃
- Moisture and Aeration
- Anaerobic metabolism is slower than aerobic metabolism
- Anaerobic metabolism produces more compounds that are toxic to a wide spectrum of microbes
- pH
- affects availability of other nutrients (e.g. N and P) needed for decomposition
- affects type of microbes present (bacteria populations decrease at low pH)
- Clay content
- clays adsorb organic matter and prevent its breakdown
- clay affects soil aeration
- Agricultural practices
- increased aeration of topsoil
- tillage results in a 60-70% reduction in soil organic due to increased, though temporarily, oxygen availability
- breakdown of biologically stable micro-aggregates
- increased soil erosion and removal (harvesting) of biomass
- Amount of biomass produced - - function of climate
- Improves aggregation and soil structure stability
- High water holding capacity (4-5 times greater than silicate clays on a mass basis)
- Erosion control and improved infiltration (rate at which water enters the soil)
- Nutrient retention - large CEC (100 to 400 cmolc kg¯¹)
- pH buffering (release and retention of H⁺ by functional groups; COOH ⇄ COO¯ + H⁺)
- Chelation of trace elements may increase availability of micronutrients
- Nutrient source via decomposition/mineralization (especially N, P and S)
- Temperature mulch - keeps the surface temperature more constant and maintains a higher water content
- Mineralization
- The conversion of an element from an organically bound form to an inorganic state–ionic form–as a result of microbial decomposition. Results in the release of elements such as Ca, Mg, K, Fe, Zn, P & N.
Evolve C as CO₂ to lower C/N ratio (respiration) and thus effectively concentrate the N
Find more N (compete with the plants for available N) to lower C/N ratio equal to that in their tissues
The rate of decomposition: will remain high as long as both organic carbon and nitrogen are available. If nitrogen should become limiting, then the decomposition rate will slow because there is not enough nitrogen for continued growth of microorganisms.
Availability of soil nitrogen: When composting with an organic material having a high C/N ratio, the microorganisms will outcompete the plants and consume all the available nitrogen (NH₄⁺ and NO₃¯) resulting in a nitrogen deficiency for the plants.
Importance of Organic Matter in Soils
| Organic material | C/N Ratio |
|---|---|
| Sawdust | 400:1 |
| Wheat straw | 100:1 |
| Legumes | 20-30:1 |
| Most soils | 10-20:1 |
| Humus | 10:1 |
| Microorganisms | 10:1 |
If the C/N ratio is higher than that of microorganisms (10:1), the microorganisms have two options:
Consequences of C/N ratios
Reference
Tisdall, J. M., and J. M. Oades. 1982. Organic matter and water stable aggregates. J. Soil Sci. 33:141–163.
Additional Resources
Bot, A. and J. Benites. 2005. The Importance of Soil Organic Matter - Key to drought-resistant soil and sustained food production. FAO Soils Bulletin 80. http://www.fao.org/3/a0100e/a0100e00.htm#Contents.