Mineralogy
© 2007 Donald G. McGahan (aka soilman) All Rights Reserved
Soil Mineralogical Properties
Soil chemical properties are regulated primarily by the clay fraction. In some soils, the clay may be a small fraction of the total volume, but it is still the most important component.
Important properties of clays
- Size
- extremely small size (<2-microns or <0.002 mm)
- termed colloid (ℹ)
- can only be seen with an electron microscope
- Surface Area
- extremely high surface area
- ranges from 10 to >800 m2/g
- Surface Charge
- most clays have a negative charge; a few clays have a small positive charge
Types of clay minerals
- Layer silicate clays
- Iron and aluminum oxides/hydroxides
Layer Silicate Clays
- Crystalline minerals with a layer-like structure.
- Each clay particle is made up of a series of layers much like the pages of a book.
- They are the dominant type of clay minerals in weakly to moderately weathered soils, such as young soils and soils of the temperate region.
Layer silicate clays are composed of two Types of horizontal sheets:
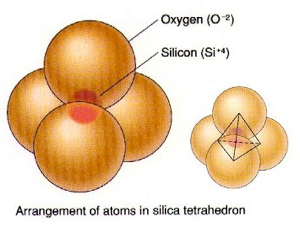
- Tetrahedral sheet
- Composed of one silicon (Si) atom surrounded by four oxygens (0).
- These individual tetrahedron link together to form a sheet.
- Octahedral sheet
- An aluminum (Al) or magnesium (Mg) is surrounded by six hydroxides (OH) forming an 8 sided building block (octahedron).
Mineral Type Groupings
- 1:1 - Type Minerals
- Composed of one tetrahedral sheet bonded to one octahedral sheet (e.g , T-O).
- Adjacent 1:1 units are bonded to each other by hydrogen bonding.
- Individual layers do not expand or contract with wetting and drying; cations cannot penetrate into the interlayer space (i.e., the space between adjacent 1:1 units). Examples: Kaolinite, Halloysite, Serpentine minerals.
- 2:1 - Type Minerals
- Composed of one octahedral sheet sandwiched between two tetrahedral sheets (e.g., T-O-T).
- Individual sheets may be WEAKLY or STRONGLY held together by mutual attraction of cations.
- Individual layers expand and contract with wetting and drying if the adjacent sheets are WEAKLY attracted.
- These clays are responsible for the large cracks in soils when they are dry. Example: Smectite.
- Individual layers DO NOT expand and contract with wetting and drying if the adjacent sheets are STRONGLY attracted.
- These clays are far less participatory in soil expansion and contraction. Examples: Micas.
- Intermediate between clays that have a strong charge and those that have a weak charge are Vermiculites. They can be considered transitory between high charge and lower charge.
- 2:1:1 - Type Minerals
- Composed of one octahedral sheet sandwiched between two tetrahedral sheets with another octahedral sheet between two sets of 2:1 (e.g., T-O-T O T-O-T).
- The coordinating cation for the hydroxide sandwiched sheet is iron, magnesium, or aluminum.
- 2:1:1 are non-expandable because of hydrogen bonding to the adjacent 2:1.
- Composed of iron or aluminum combined with oxygen (O) or hydroxides (OH).
- The dominant minerals of highly weathered soils in the tropics, semi-tropics, and old soils.
- Dominated by iron and aluminum minerals.
- Gibbsite (Al(OH)3) = bauxite ore, mined as a source of aluminum.
- Hematite (Fe2O3)
- Goethite (FeOOH) = mined as a source of iron ore.
- Incongruent weathering
- formed by alteration of primary or secondary minerals. (Alteration)
- Precipitation from soil solution
- products of weathering accumulate in the soil solution and may exceed the solubility product of certain minerals.
- Primary minerals
- minerals that have not been altered chemically since they solidified from the molten magma.
- Secondary minerals
- recrystallized or modified products from the chemical breakdown and/or alteration of primary minerals (clay minerals are an example of secondary minerals: Soils are clay factories!).
- fertility
- physical properties
- the direction of further soil development
- the rate of further development
- Exchangeable Ions
- Ions (charged atoms or molecules) held by electrical (coulombic) attraction at charged surfaces; can be displaced by exchange with other ions. ℹ
- Cations
- ions which have a positive charge and are attracted to negatively charged sites or solids.
- Anions
- ions which have a negative charge and are attracted to positively charged sites or solids.
- Cation exchange capacity (CEC)
- The total amount of positive ions (cations) that a soil can adsorb exchangeably. ℹ or equivalently the number of negative charges held by coulombic attraction (ℹ). Each negative charge balances one charge from a cation charge.
- Anion exchange capacity (AEC)
- The amount of exchangeable anions per weight of dry soils or equivalently the number of positive charges held by coulombic attraction (ℹ). Each positive charge balances one charge from a anion charge.
- The higher the pH, the greater the negative charge (CEC).
- The lower the pH, the greater the positive charge (AEC).
- Humus (ℹ) has either no charge or negative charge; it generally can be considered to not develop positive charge.
- Kaolinite I
- Al₄Si₄O₁₀(OH)₈
- Kaolinite II
- Al₄(Si₃.₈₇Al₀.₁₃)O₁₀(OH)₈
- Montmorillonite
- (Al₃Mg)Si₈O₂₀(OH)₄
Oxides and Hydroxides
Relative Stability of Common Clay Minerals
Oxide/Hydroxides > 1: 1 layer silicates > 2: 1 layer silicates > primary minerals (except quartz)
Formation of Clay Minerals
The creation and alteration of clay sized minerals in soils is dynamic. Additional information about the clay size fraction and clay mineral formation and alteration is discussed in the Classification and Chemical sections of this reader. Briefly two main forms are:
Soil Minerals
Soil organic matter (SOM) is very reactive in soil and much of it is small, colloidal sized, termed humus, but humus is not mineral.
Soil start formation from parent material; mostly. These parent materials, when inorganic, are to the overwhelmingly greater extent crystalline minerals.
The minerals of the ‘parent material’ are minerals inherited from a system that is quite different from near the earth surface where soils live. Most of these minerals formed, and were at equilibrium, at greater heat and/or greater pressures than where soils now live (lower pressure and temperature).
Defining weathering processes in more detail deserves a separate treatment. For now, it is sufficient to say that the ‘parent material’ minerals have some impetus to become some other minerals. What minerals they become are called secondary minerals and secondary minerals are more stable at the lower
The inherited minerals in parent material can be made up of many different combinations of elements. The most common elements for parent material minerals and secondary minerals are Oxygen (O) and Silicon (Si), and not surprisingly, they are common in the minerals commonly found in soils. Rocks are made of crystalline minerals and minerals are made up of atoms.
Some Simple Math
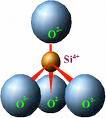
The silicon atoms are positively charged (+4) cations. The oxygen atoms are negatively charged (–2) anions. The silicon and the oxygen combine to form a unit called a silicon tetrahedron. Tetra for four. The shape is a pyramid with four sides. The silicon is at the center surrounded by four oxygens. The cation at the center of the formative tetrahedron unit is called the coordinating cation.
One Si (+4) = +4
Four O (–2) = –8
Add them and you have a charged tetrahedron (the charge is –4): SiO₄⁴⁻
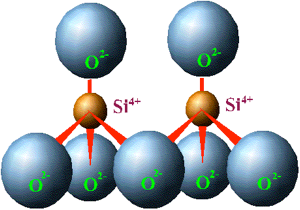
By sharing oxygens in the arrangement of a tetrahedron with a neighboring tetrahedron, the negative charge on each tetrahedron can be reduced. The following diagram has two tetrahedrons sharing an oxygen, therefore, the charge on the entire compound is now –6, or –3 for each tetrahedron.
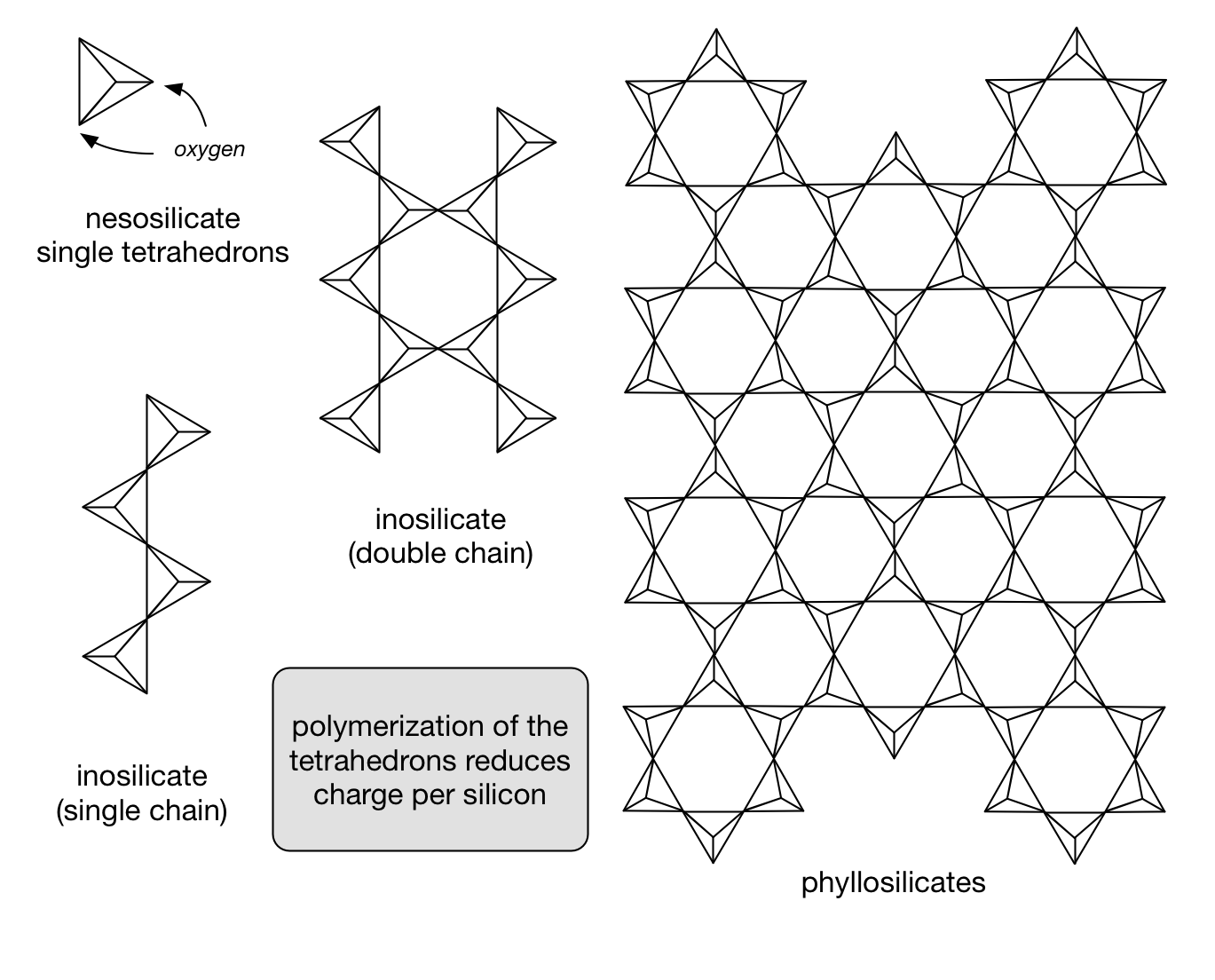
Minerals can be grouped together by such linkages or arrangement. The extent of the repeating order of the atoms determine the size of the crystal.
While a great number of minerals have silicon and oxygen as their backbone, the arrangement of these atoms varies as do the other elements making up the mineral(s).
Polymerization goes along way to accounting for charge balancing, but to balance charge other accessory cations can be included in the minerals. The accessory cations and the various oxygen sharing arrangements have implications on how resistant the primary mineral is to being altered into a secondary mineral, and when altered what cations are released into solution. Some of the released cations are nutrients for biologicals and therefore impact plant growth. Accessory minerals that balance charge may be more easily exchanged for another accessory mineral.
The kinds of minerals in a soil influences the soils:
Some of the more resistant primary minerals, that is resistant to weathering and becoming some other secondary mineral(s), are often concentrated in the sand and silt soil separates.
Following are links to external sources. This information in the external source course is content that would have been presented in primary school science courses. Go through the following learning modules by selecting the links listed below. These modules are not specifically about soil minerals and are a little more in-depth about some non-soils related issues, but they are good. Use the book and lectures as a guide of ’what your responsible for in this class.
Scroll down to Mineral Definitions by Anne E. Egger[1]
Mineral Properties by Anne E. Egger[2]
Minerals III: The Silicates by Anne E. Egger[3]
Hint: The questions & quizzes at the end of each section can be taken and repeated until mastery is demonstrated. These are external links.
Soil chemical properties are regulated primarily by the clay fraction. In some soils, the clay may be a small fraction of the total volume, but it is still the most important component.
Solid-Liquid Interactions
Electrostatic interactions where ions from solution interact with the solids at the surfaces of the solid particles, both inorganic and organic, are some of the most important and well understood. While interactions can happen in the air, most happen when the solution is bathing the solids.
Other interactions occurring include precipitations from a soluble phase, dissolutions of solids, and specific adsorptions are also among solid-liquid reactions studied to explain soil genesis and soil functions.
When the reaction is electrostatic and an ion is replaced by other ions this is “ion exchange.” Previously in the discussion of the building blocks of phyllosilicate minerals octahedrons and tetrahedrons were introduced. The coordinating cation of these building blocks can sometimes be substituted with another cation with a lower charge. This results in a net negative charge of the phyllosilicate mineral. There is generally more solids with a net negative charge than positive charge.
The resultant electrostatic, or coulombic, attraction holds the ions, but this is weaker than covalent bonding and it is less specific.
Cation Exchange Capacity
The soils ability to hold cations in this electrostatic attraction includes both the phyllosilicate (clays) and the humus (organics) and is consequently referred to as the “cation exchange complex (ℹ:).”
This exchange capacity is important because exchangeable cations are available to plants and can supplement the cations removed from solution, and they are held against loss with waters percolating downward.
How do soil materials develop charge?
pH Dependent Charge
Mineral edges have broken bonds that can develop a charge.
Oxides/hydroxides have OH groups on their surface that can develop positive or negative charges depending on the solution pH.
\[ M - OH_2^ + \underset{{ + {H^ + }}}{\overset{{ - {H^ + }}}{\longleftrightarrow}}M - O{H^0}\underset{{ + {H^ + }}}{\overset{{ - {H^ + }}}{\longleftrightarrow}}M - {O^ - } \]
where: M = a metal such as Al
\[ \text{Low pH} \longleftrightarrow \text{High pH}\]
Permanent Charge
Isomorphous substitution (ℹ) may result in either positive or negative charge. The substitution of one cation for another within the crystal structure may lead to a charge imbalance. Usually this results in a net negative charge on the mineral. Occurs primarily in layer silicate clays. (Ion charge: Si4+, Al3+, O2-)
| Tetrahedral sheet | |
|---|---|
| no substitution | (Al3+ substitution for Si4+ ) |
| Si2O4 | SiAlO4- |
| No charge | One excess negative charge |
Above it was established that the primary unity of the tetrahedral sheet is Si₂O₄.
While this is simple accounting it is important to keep on task. When determining the charge on a phyllosilicate mineral, use a periodic table to find the atomic charge for each element, and lay out a table similar to the following table to determine the charge on a mineral.
| No. of atoms | Atom | Atomic charge | Layer charge |
|---|---|---|---|
| 4 | Al | +3 | +12 |
| 4 | Si | +4 | +16 |
| 10 | O | –2 | –20 |
| 8 | OH | –1 | –8 |
| Balance | 0 |
The above Kaolinite has no net charge, but consider substitution of Al³⁺ for Si⁴⁺ for one out of every eight of these units. For clarity in understanding the following formula shows the Si and Al that are tetrahedral coordinating cations are grouped together in parentheses.
| No. of atoms | Atom | Atomic charge | Layer charge |
|---|---|---|---|
| 4.13 | Al | +3 | +12.39 |
| 3.87 | Si | +4 | +15.48 |
| 10 | O | –2 | –20 |
| 8 | OH | –1 | –8 |
| Balance | –0.13 |
The kaolinite II above has a net negative charge of 0.13 and the loci of the substitution is in the tetrahedral sheet.
Isomorphous substitution is not limited to only the tetrahedral sheet. More often it happens in the octahedral sheet.
| Octahedral sheet | Octahedral sheet |
|---|---|
| (no substitution) | (Mg²⁺ substituted for Al³⁺ |
| Al(OH)₆ | Mg₀.₃₃Al₀.₆₇(OH)₆ |
| No charge | One excess negative charge |
Now look at a different clay mineral. The following is a 2:1 group type and is a smectite called montrorillonite. Try your hand at the accounting.
| No. of atoms | Atom | Atomic charge | Layer charge |
|---|---|---|---|
| 4 | Al | +3 | +12 |
| 1 | Mg | +2 | +2 |
| 7 | Si | +4 | +28 |
| 20 | O | –2 | –40 |
| 4 | OH | –1 | –4 |
| Balance | –2 |
Your turn to practice.
Choose from the following a try out the above method.
Table of formulas for clay minerals.
| Mineral | Formula |
|---|---|
| Kaolinite (1:1) | Al₄Si₄O₁₀(OH)₈ |
| Halloysite | Al₄Si₄O₁₀(OH)₈·4H₂O |
| Smectite (2:1) | |
| Bentonite | (Fe₁.₃₃Mg₀.₆₇Al₂Si₈O₂₀(OH)₄ |
| Montmorillonite | (Al₃Mg)Si₈O20(OH)₄ |
| Nontronite | Fe₄(Si₇Al)O₂₀(OH)₄ |
| Saponite | Mg₆(Si₇Al)O₂₀(OH)₄ |
| Vermiculite (2:1) | Mg(Al, Fe, Mg₄)(Al₂Si₆)O₂₀(OH)₄·nH₂O |
| Chlorite (2:1:1) | Mg₆(OH)₁₂(Al, Mg₅)(Al₂Si₆)O₂₀(OH)₄ |
The iron (Fe) in the above formulas is in the ferrous ionic state (Fe2+).
The excess charge on the mineral or organic matter is balanced by attracting cations (or anions) to the mineral surface. These ions are termed exchangeable cations (ℹ) (or anions) and are subject to replacement by other cations (or anions).
Measuring cation exchange capacity and exchangeable cations: :Expressed in cmolc/kg (centimoles of charge) for CEC or AEC. (1 cmol Al3+ per kg soil = 3 cmolc/kg)
Recall that 1 mol = 100 cmol just as 1 mol = 1,000 mmol. Moles of charge (molc) relationships are the same where 1 molc = 100 cmolc just as 1 mol = 1,000 mmolc.
What determines which cations are on the cation exchange capacity?
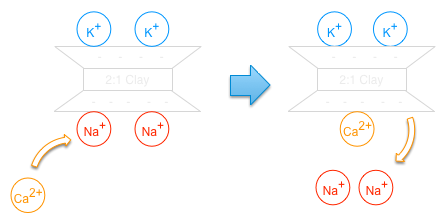
Cation selectivity: Coulomb’s Law states that ions having a smaller hydrated size and greater charge are more strongly attracted to the exchange sites. Given equal concentrations of each cation in the soil solution, their affinity for the exchange site follows:
Al3+ > Ca2+ > Mg2+ > K+ ~ NH4+> Na+
| Ion | Charge | Radius (nm) nonhydrated | Radius (nm) hydrated | Atomic Weight* (g) | Equivalent Weight # (g) |
|---|---|---|---|---|---|
| Al | +3 | 0.050 | - | 27 | 9 |
| Ca | +2 | 0.099 | 0.300 | 40 | 20 |
| Mg | +2 | 0.065 | 0.400 | 24 | 12 |
| Na | +1 | 0.095 | 0.215 | 23 | 23 |
| K | +1 | 0.133 | 0.155 | 39 | 39 |
| H | +1 | - | 0.455 | 1 | 1 |
| O | –2 | 0.145 | - | 16 | 8 |
| * the mass of one mole of atoms | |||||
| # The mass necessary to exchange 1 mol of charge, which is equivalent to 1 mole of hydrogen | |||||
| where nm is nanometers or 1 x10–9 m: g is grams | |||||
Cation concentration: the concentration of exchangeable cations depends on the concentration of the cations in the soil solution. Thus, if sodium is high in the soil solution, it will be high on the CEC.
Base Saturation%: the extent to which the exchange capacity is saturated with Ca, Mg, K and Na
$$\text{Base Saturation %}= \left(\frac{ \text{Ca}^{2+} + \text{Mg}^{2+} + \text{K}^+ + \text{Na}^+}{\text{Cation Exchange Capacity}}\right) \times 100$$
| Texture | CEC (cmolc kg–1 soil) |
|---|---|
| sandy loam | 2–15 |
| clay | 5–60 |
| organic matter | 200–400 (all pH dependent) |
| Mineral Group | CEC (cmolc kg–1 soil) |
|---|---|
| 1:1 - Kaolinite | 3–15 |
| 2:1 - Smectite | 90- 120 |
| 2:1- Vermiculite | 100–150 |
| Oxides/hydroxides | low; pH dependent; often positive below pH 7 |
CEC : organic matter > 2:1 layer silicates > 1:1 layer silicates > oxides/hydroxides
AEC : oxides/hydroxides >> 1:1 layer silicates > 2: 1 layer silicates
CEC varies with the pH. The higher the pH, the higher the CEC and the lower the AEC.
The excess charge on the mineral or organic matter is balanced by attracting cations (or anions) to the mineral surface. These ions are termed exchangeable cations (or anions) and are subject to replacement by other cations (or anions).
A household example is water softeners as they are ion exchangers composed of synthetic resins saturated with Na+:
2Na+-resin + Ca2+ ↔ Ca2+-resin + 2Na+
Calcium and magnesium produce hard water; sodium produces soft water.
Clays are also reactive because of the enormous amount of surface area.
How many M² comprise a soccer field? (Where M is meter.)
How many M² comprise a football field?
Below is a table that compares some surface area values for common clay size particles.
| Surface area varies with the type of clay | 10³ M² kg⁻¹ |
|---|---|
| 1:1 − Kaolinite | 10–20 |
| 2:1:1 - Chlorite | 70–150 |
| 1:1 - Mica | 70–120 |
| 2:1 − Smectite | 600–800 |
| 2:1 − Vermiculite | 600–800 |
| Humus | 900 |